Griffith University researchers are behind some major breakthroughs on the micro and nano scale, with recent research finding that getting more detailed breakdowns on wearable device owners’ overall health stats could soon become a reality.
Dr Jun Zhang and Dr Navid Kashaninejad from Griffith’s Queensland Micro- and Nanotechnology Centre conduct research on two current challenges of a field known as microfluidics: biosampling through the separation of nanoparticles; and wearable technologies.
The two researchers have published their outcomes on this work, under the mentorship of QMNC Director Professor Nam-Trung Nguyen, who is renowned for his research in micro elastofluidics.
What is micro elastofluidics?

Professor Nam-Trung Nguyen.
Micro elastofluidics uses bendiness and stretchiness to enable fluid handling that leads to faster and more accurate diagnosis of health conditions.
“This technology allows for precise handling of small amount of samples and reagents,” Professor Nguyen said.
“My research has been aiming at medium-term impact and has moved from sensors for cars to shrinking down a lab onto a chip to wearable devices for health monitoring.”
For instance, the QMNC research team has developed a range of cell stretching devices for studying cell behaviour under mechanical stress. Ongoing works are on skin models, skin cancer models, gut models and blood vessel models.
“As a complement to this fundamental research, I’ve recently established the field called ‘micro elasofluidics’ that uses stretchability and flexibility from molecular scale to device scale for better handling of liquid samples,” Professor Nguyen said.
“It integrates microfluidics into flexible wearable and implantable devices.”
Benefits of separating nanoparticles
Dr Zhang’s recent research has focused on the separation of nanoparticles and how they can be used to gather information on different health conditions.
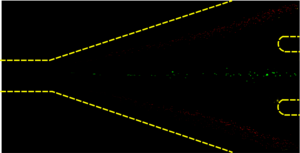
The separation of two particles, with the red and green indicating their separation in microfluidic channels.
These nanoparticles can be extracellular ‘vesicles’, which are particles secreted by cells in our body as a way to communicate.
“Extracting these particles from liquid samples such as blood would provide a wealth of information on health conditions,” Dr Zhang said.
“The same particles may have anti-inflammation and anti-aging effects and potentially serve as therapeutics against aging and age-related diseases such as Alzheimer’s.”
The challenge is to separate the particles without damaging the delicate surface. Current techniques such as ultracentrifugation and ultrafiltration often damage these particles.
Dr Zhang’s method uses a unique fluid physics phenomenon called ‘inertial microfluidics’ to avoid damaging these useful but delicate particles.
“Advances in microfluidic technologies have made a significant impact on the biomedical field,” Dr Zhang said.

Dr Jun Zhang.
“Microfluidic technologies offer an unprecedented capability to precisely control the motion of fluids and the dissolved or suspended analytes at microscale.”
“These analytes could be various blood cells, bacteria, virus, DNA, RNA, proteins, glucose, lipid etc. And precisely manipulating and detecting these tiny particles is critical for disease diagnosis and monitoring health conditions in the human body.
“We have recently been invited to publish a critical review in the prestigious journal of Lab on a Chip.
“We compared the traditional methods and microfluidics on separating nanoparticles in this paper. The conventional techniques generally possess the advantages of time efficiency, high yield, ease of use and good reproducibility, but they are limited by the high cost and low purity. Microfluidic technologies are superior in enhanced separation resolution and more cost-effective.
“Over the past decade, significant progress has been achieved in microfluidic technology for separating nanoparticles with a broad range of applications, however, there is still a considerable gap to be filled. We have explored the challenges and solutions to these limitations that are often overlooked.”
Biosamples from wearable devices?

Dr Navid Kashaninejad.
Dr Kashaninejad’s research explores the collection of health condition information via wearable devices, using the concept of micro elastofluidics.
Currently, wearable devices such as smart watches can only collect physical information from the wearer. Collecting information about biochemical and biological conditions is much more difficult because of the lack of ‘fluid handling’ capability of these devices.
Dr Kashaninejad’s research explores simple, practical solutions for liquid handling on flexible and skin-conformal surfaces so that solutions for sampling sweat and subdermal body fluids can be easily investigated.
“We will find these solutions in the near future in wearable devices that can provide more information about the health condition of the wearer than the current smartwatch,” he said.
“On-skin wearable systems for biofluid sampling and biomarker sensing can revolutionise the current practices in healthcare monitoring and personalised medicine.
“However, there is still a long path toward complete market adoption and acceptance of this fascinating technology.
“My recent paper compared various microfluidic platforms that could be used for on-skin wearable devices. These platforms include semiconductor-based, polymer-based, liquid metal-based, paper-based, and textile-based microfluidics.
“These platforms can enhance the stretchability of on-skin wearable biosensors at the device level, and there are potential microfluidic solutions for collecting, transporting, and controlling the biofluids.
“The application of finger-powered micropumps is a viable solution for precise and on-demand biofluid pumping.
“There is great potential in this field, such as the applications of stretchable continuous-flow micro elastofluidics, stretchable superhydrophobic surfaces, liquid beads — tiny capsules with liquid content – as a form of digital micro elastofluidics, and topological liquid diodes that receive less attention but have enormous potential to be integrated into on-skin wearable devices.”
